Back تجربة غير سريرية Arabic Investigació preclínica Catalan Desarrollo preclínico Spanish 非臨床試験 Japanese Доклиническое исследование Russian
This article needs more reliable medical references for verification or relies too heavily on primary sources. (June 2020) |  |
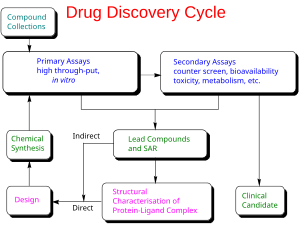
In drug development, preclinical development (also termed preclinical studies or nonclinical studies) is a stage of research that begins before clinical trials (testing in humans) and during which important feasibility, iterative testing and drug safety data are collected, typically in laboratory animals.
The main goals of preclinical studies are to determine a starting, safe dose for first-in-human study and assess potential toxicity of the product, which typically include new medical devices, prescription drugs, and diagnostics.
Companies use stylized statistics to illustrate the risks in preclinical research, such as that on average, only one in every 5,000 compounds that enters drug discovery to the stage of preclinical development becomes an approved drug.[1][2]
- ^ Emanuel EJ (9 September 2015). "The Solution to Drug Prices". New York Times.
On average, only one in every 5,000 compounds that drug companies discover and put through preclinical testing becomes an approved drug. Of the drugs started in clinical trials on humans, only 10 percent secure F.D.A. approval. ...
- ^ "Drug Approvals - From Invention to Market...12 Years!". MedicineNet. Retrieved 2021-04-21.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search